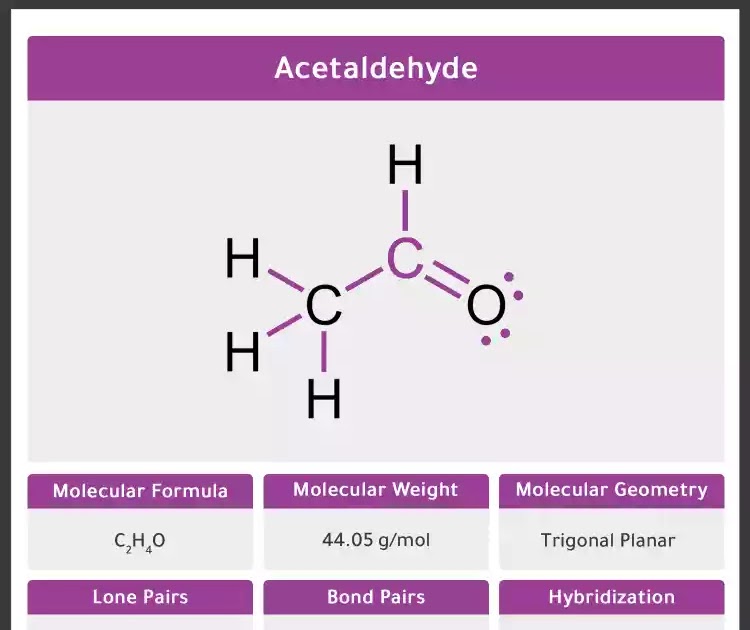
Acetaldehyde (Ethanal) CH3CHO Molecular Geometry Hybridization
1. trigonal planar. 2. sp. 2. linear. So, for the two-dimensional molecule drawings below, Give the hybridization of all non-H atoms; Re-draw the molecules to reflect a possible 3-D geometry.

CH3COOH acetic acid molecule Stock Vector & Stock Photos Bigstock
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Draw the Lewis structure of acetaldehyde (CH3CHO) and then choose the appropriate pair of molecular geometries of the two central atoms. Your answer. choice is independent of the orientation of your drawn structure.

Solved Draw Lewis structure(s) showing all possible
Hello everyone, welcome back to Geometry of Molecules, where we make chemistry fun and easy. For today's video, we are going to share our step-by-step proces.

SOLVED Draw the Lewis structure for each organic compound from its
Acetaldehyde (CH3CHO) -Ethanal (common name acetaldehyde) is an organic compound with the formula CH3CHO. Acetaldehyde is one of the most frequently found air toxins with cancer risk greater than one in a million. Visit BYJUS to study the uses, preparations, properties, and structure of acetaldehyde (C2H4O) explained by the chemistry experts.

CH3OCH3 Lewis Structure How to Draw the Lewis Structure for CH3OCH3
For each of the following structures, Draw a Lewis structure; fill in any nonbonding electrons. Calculate the formal charge on each atom other than hydrogen d. [ (CH3)3O]+ e. CH3NC f. (CH3)4NBr. Draw a line-angle formula for each compound. c. H2CHCH (OH)CH2CO2H d. CH2CHC (CH3)CHCOOCH3.
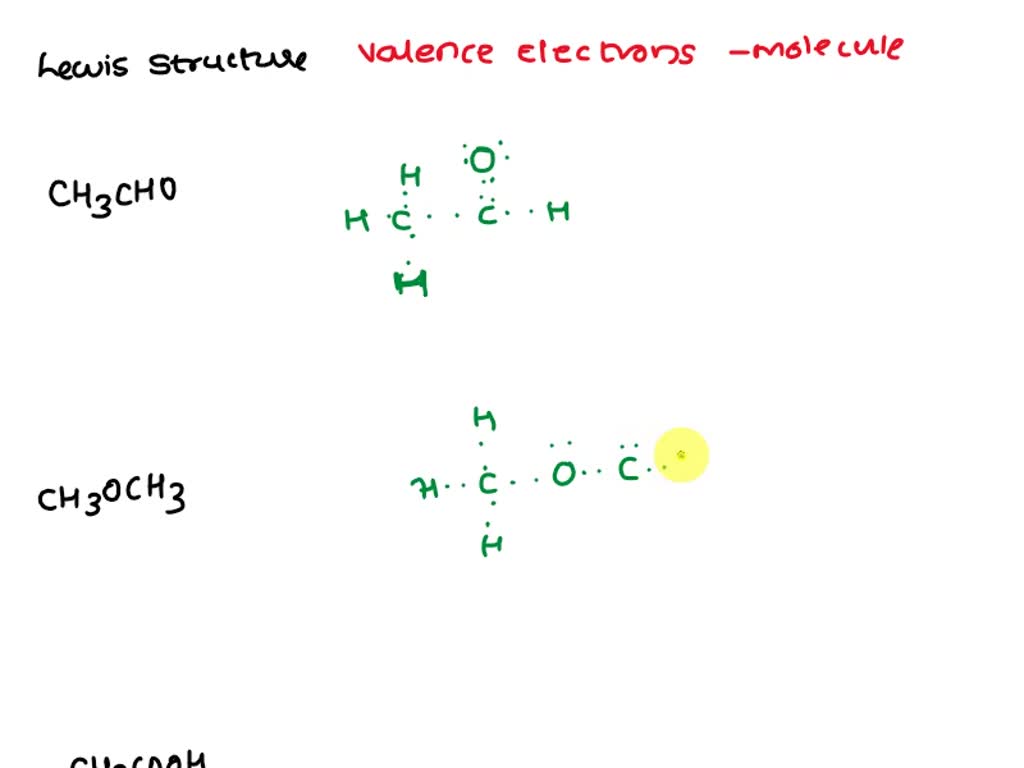
SOLVED Draw a Lewis structure for each of the followinga. CH3CHO b
To properly draw the CH 3 CHO Lewis structure, follow these steps: #1 Draw a rough sketch of the structure. #2 Next, indicate lone pairs on the atoms. #3 Indicate formal charges on the atoms, if necessary. #4 Minimize formal charges by converting lone pairs of the atoms.
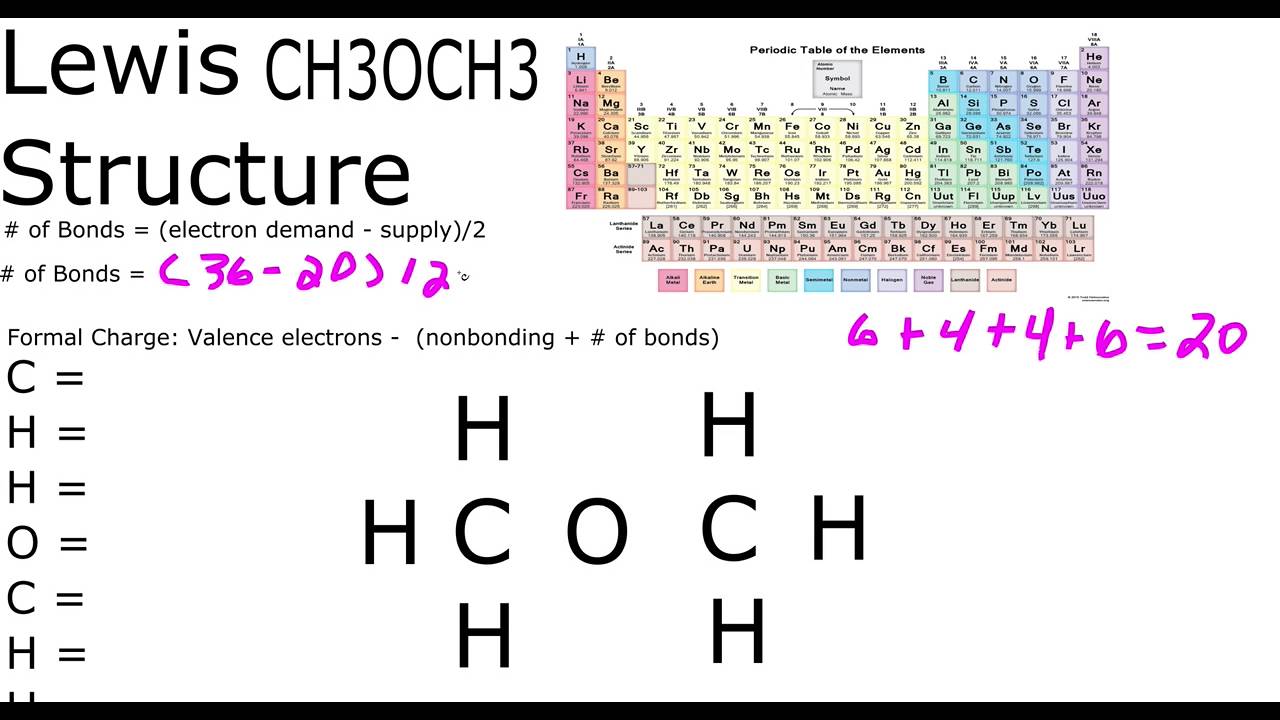
CH3OCH3 Lewis Structure YouTube
In the Lewis structure, each hydrogen has a zero placed nearby while the nitrogen has a +1 placed nearby. Adding together the formal charges on the atoms should give us the total charge on the molecule or ion. In this case, the sum of the formal charges is 0 + 1 + 0 + 0 + 0 = +1. Exercise 8.5.2 8.5. 2.
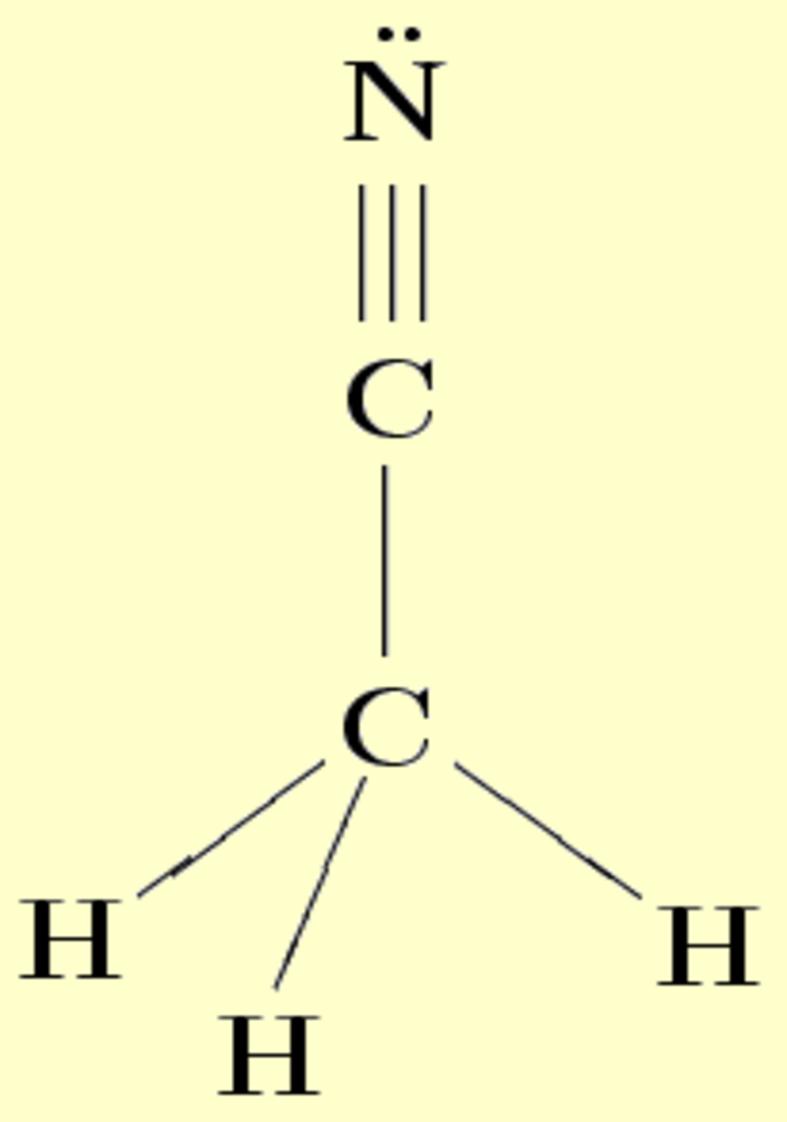
Lewis Diagram For Ch3cn
I quickly take you through how to draw the Lewis Structure of CH3CHO (ethanal). I also go over hybridization, shape, sigma, pi bonding and bond angles.

Chemistry Class 11 NCERT Solutions Chapter 4 Chemical Bonding and
Draw Lewis structure(s) for the acetaldehyde molecule (CH3CHO). If there are equivalent resonance structures, draw all of them. - Draw one structure per sketcher box, and separate added sketcher boxes with the â†" symbol. - Do not include overall ion charges or formal charges in your drawing. - Do not draw double bonds to oxygen unless.
Solved Draw a correct Lewis structure for acetaldehyde,
A step-by-step explanation of how to draw the CH3CH3 Lewis Dot Structure.For the CH3CH3 structure use the periodic table to find the total number of valence.

Ch3cho acetaldehyde molecule Royalty Free Vector Image
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH 3 CHO, sometimes abbreviated as MeCHO. It is a colorless liquid or gas, boiling near room temperature. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry.Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is.

CH3CHO Lewis Structure (Acetaldehyde) YouTube
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

SOLVED Predict all bond angles and the shape of the molecules and ions
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.

How to Draw the Lewis Dot Structure for CH3CH3 YouTube
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
CH3COO Lewis Structure How to Draw the Lewis Structure for CH3COO
CH3OCH3 Lewis Structure. Lewis Structure is the initial step towards finding out about the chemical bonding in a given molecule. It deals with the valence or the outermost shell electrons which come together in pairs and form covalent bonds between atomic elements. We use electron dot notations to represent the valence electrons during the.
Lewis structure, Hybridization, and Molecular Geometry of CH3OH by
Acetaldehyde | CH3CHO or C2H4O | CID 177 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities.